Contents
Editor-in-Chief, Prof Massimo Colombo MD
María Reig M.D. PhD and Jordi Bruix M.D.,
Barcelona Clinic Liver Cancer (BCLC) Group,
Liver Unit, Hospital Clinic Barcelona, IDIBAPS,
University of Barcelona. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd),
Barcelona, Spain.
Zoe Mariño M.D. and Xavier Forns M.D.,
Liver Unit, Hospital Clinic, IDIBAPS,
University of Barcelona. CIBERehd,
Barcelona, Spain.
Q. Professor Bruix, has the advent of HCV DAA met the expectations of patients and physicians?
The advent of effective and safe therapies for hepatitis C virus infection has been eagerly awaited for decades by patients and physicians alike. This therapeutic development has come about in the form of the new Direct Acting Antivirals (DAA) and their benefit was immediately expected to change the field of liver diseases(1). It was soon envisaged that the health policies already in place to prevent viral transmission together with the DAA therapies would almost completely eradicate HCV and would prevent infected individuals from transition into chronic infection reaching ultimately end-stage liver disease and, in some cases, developing liver cancer. While these long-term goals may still hold true at a population basis, some reports have suggested that the therapeutic benefits of DAA may not be free of risks as some patients may develop unexpected events associated to DAA therapy(2-5).
Q. Are there any risks connected with DAA therapy of HCV?
This is the case with the risk of hepatocellular carcinoma (HCC) occurrence that cirrhotic patients are at risk to develop (6-9). Furthermore, even if successfully treated by resection, transplantation or ablation, the risk of recurrence of HCC during follow-up is high, and effective preventive measures are not available(6,7,9). It is worth mentioning that DAA do not have an anticancer effect themselves, but rather diminish or eliminate the oncogenic damage that is key for cancer development. Hence, even if effective against HCV, the already existing cancer clones (primary or disseminated from a first already treated HCC) are not eliminated by DAA therapy. Thus, the risk of cancer will persist in those who have already acquired the risk (namely cirrhotic patients) and in those who have been diagnosed and successfully treated for HCC. Therefore, despite DAA therapy, some patients will still encounter this clinical event.
Q. Does this mean we should avoid or defer treatment of HCV in patients at risk of liver cancer?
What has become a major concern is whether the risk is increased rather than reduced after treatment. Several physicians have seen cases of HCC emergence soon after DAA treatment, but it was unfeasible to figure out whether these observations were over-reported or if such findings had a major emotional impact and thus flawed the perception of the magnitude of the problem. The first structured report concerning a potential increase of cancer with a close time association with DAA therapy came from Barcelona(6). In this study, we reported that the incidence of HCC recurrence was unexpectedly high immediately after DAA treatment (27.6%). The striking time association resulted in raising an alarm signal through a manuscript that illustrated all clinical and tumoral data so that readers could scrutinize the data in full to permit all kinds of further analysis.
Q. How frequent is HCC recurrence in DAA treated patients?
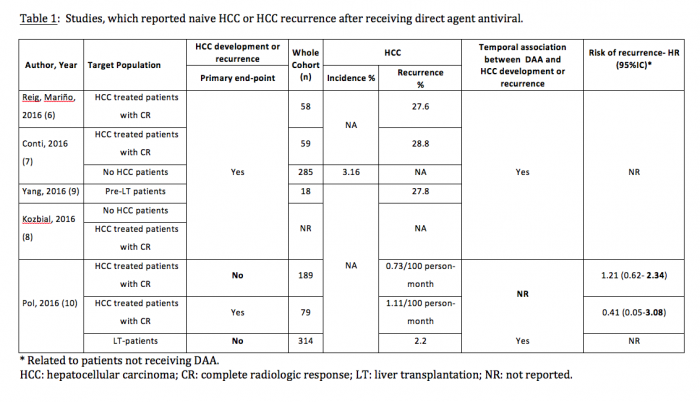
The study described patients that had been treated with DAA after securing complete response following HCC treatment and had a 41.17% likelihood of presenting HCC recurrence in the first 4 months following treatment with DAA. This was independent of the antiviral treatment regime and response. These findings raised a major concern and have primed several groups to analyse their experience aiming to refute(10,11) or validate(7,9) the Barcelona findings both in patients who had had HCC successfully treated and in patients without such prior diagnosis(8) (Table 1). The results of such studies have to be carefully reviewed in order to understand if HCC screening was in place and performed properly in treated patients, if the follow-up included screening and for how long after treatment, and ultimately, if HCC incidence during follow-up was even one of the end-points of the study. Indeed, some of the studies that have suggested that there is no increased risk of HCC after DAA therapy may lack some of these important parameters(10).
Q. Has anyone else reported an increased risk of HCC in DAA treated patients?
To date, confirmatory reports about increased HCC recurrence after DAA therapy and striking time association have also been published by groups from Italy(7) and from the USA(9), while studies from France in several cohorts of patients have not registered such an increase. However, a clear time association between antiviral treatment and HCC recurrence has been reported in transplanted patients(10). It is important to note that the French cohorts(10) are a subgroup analysis of subgroups of patients of larger cohorts, and in them, the HCC surveillance, follow-up length and primary end-points of the primary studies were not optimal to capture HCC recurrence. Interestingly, a French cohort that has been prospectively followed for several years described that the HCC incidence after DAA decreases after 12 months of successful therapy. However, the incidence during the first year in the study was around 6%, which is clearly in excess of the incidence that was predicted in the same cohort (1.9%) when DAA were not available. Hence, it may be that what has been observed is an unexpected increase after treatment, while later on, the risk would have returned to baseline. The description of the incidence within the 6-12 months prior to DAA would have allowed a clearer understanding of these results. Intriguingly, another cohort from Austria also reports a 6% incidence in naïve cirrhotic patients in the first months following treatment(8).
Q. What needs to be done to solve this clinical dilemma?
Results related to HCC incidence from the cohorts of treated patients in the DAA registration trials, and of investigator driven studies assessing HCC incidence, as a primary end-point should be reported early and the concern should be clarified. Such studies will have to stratify the data according to gender, age and presence/absence of cirrhosis, alcohol consumption and portal hypertension. All these data will finally refute the concern or consolidate it and raise the need to establish risk-benefit decision-making policies to guide the optimal time for treatment initiation.
Q. Is there any explanation for the link between DAA therapy and increased HCC occurrence?
A relevant aspect to investigate in this situation is what could be the mechanism for an increased cancer emergence (primary or metastatic) after DAA therapy. The most likely one is the immune distortion due to the rapid viral load reduction that abruptly changes the inflammatory profile of the infected liver and of the entire human body, too. A sudden immune surveillance impairment may allow existing preclinical cancer clones to grow and become clinically detectable. The extent of such cancer emergence will not be homogeneous, as some patients may have few subclinical clones just within the liver, while others may have a more extensive spread as it is known that the metastatic process is very complex. If such immune impairment is real, the concern about unexpected neoplastic events should go beyond the liver cancer problem. Genomic hits accumulate during human lifetime and some tumors may remain dormant for years. However, in a transient immune failure setting such clones may progress and be diagnosed during follow-up. If this is not systematic, cancer recognition will occur when it becomes symptomatic and this will not be close in time to DAA therapy.
These observations should just serve to prime a major research effort from pharmacovigilance agencies, clinical investigators and the biomedical industry. Actions of all sorts are already ongoing on different fronts and as with all health challenges the path for a solution comes from the identification of the problem, the understanding of the pathogenic mechanism and ultimately, the development of an effective intervention to prevent its occurrence.
- EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol 2015;63:199–236. doi:10.1016/j.jhep.2015.03.025.
- Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, et al. Hepatitis B Virus Reactivation during Successful Treatment of Hepatitis C Virus with Sofosbuvir and Simeprevir. Clin Infect Dis 2015;61:1304–6. doi:10.1093/cid/civ474.
- EMA reviews direct-acting antivirals for hepatitis C. EMA Rev Direct-Acting Antivirals Hepat C 2016:http://www.ema.europa.eu/docs/en_GB/document_libra.
- Welker M-W, Luhne S, Lange CM, Vermehren J, Farnik H, Herrmann E, et al. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/sofosbuvir treatment. J Hepatol 2016;64:790–9. doi:10.1016/j.jhep.2015.11.034.
- Perelló CS, Fernández-Carrillo C, Londoño M-C, Arias-Loste T, Hernández-Conde M, Llerena S, et al. Reactivation of Herpesvirus in Patients With Hepatitis C Treated With Direct-Acting Antiviral Agents. Clin Gastroenterol Hepatol 2016. doi:10.1016/j.cgh.2016.05.016.
- Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, et al. Unexpected early tumor recurrence in patients with hepatitis C virus -related hepatocellular carcinoma undergoing interferon-free therapy: a note of caution. J Hepatol 2016. doi:10.1016/j.jhep.2016.04.008.
- Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early Occurrence and Recurrence of Hepatocellular Carcinoma in HCV-related Cirrhosis Treated with Direct Acting Antivirals. J Hepatol 2016. doi:10.1016/j.jhep.2016.06.015.
- Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with SVR following IFN-free DAA treatment. J Hepatol 2016. doi:10.1016/j.jhep.2016.06.009.
- Yang JD, Aqel BA, Pungpapong S, Gores GJ, Roberts LR, Leise MD. Direct Acting Antiviral Therapy and Tumor Recurrence after Liver Transplant for Hepatitis C-Associated Hepatocellular Carcinoma. J Hepatol 2016. doi:10.1016/j.jhep.2016.06.023.
- Pol S. Lack of evidence of an effect of Direct Acting Antivirals on the recurrence of hepatocellular carcinoma: The ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CIRVIR and CO23 CUPILT cohorts). J Hepatol 2016. doi:10.1016/j.jhep.2016.05.045.
- Torres HA, Vauthey J-N, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J Hepatol 2016. doi:10.1016/j.jhep.2016.05.034.
Prof. Jordi Bruix M.D
BCLC group. Liver Unit. IMDiM. CIBEREHD. IDIBAPS. Hospital Clínic
c/ Villarroel, 170. Escala 11, 4ª planta
08036 Barcelona, Spain
Email: [email protected]
References
Prof. Peter Ferenci M.D.,
Division of Hepatology and Gastroenterology,
Medical University of Vienna, Vienna, Austria
Q. Professor Ferenci, does antiviral therapy eliminate the risk of hepatocellular carcinoma in hepatitis C patients?
Virus elimination by interferon-based therapy decreases the incidence of hepatocellular carcinoma (HCC) in patients with advanced fibrosis/cirrhosis(1-3). However, the risk of HCC development remains even in patients with sustained virologic response, although at a much lower level(2). Even in patients without cirrhosis, HCC can occur many years after sustained virologic response (SVR)(3).
Q. What are the additional clinical benefits we can expect with all oral DAA therapy?
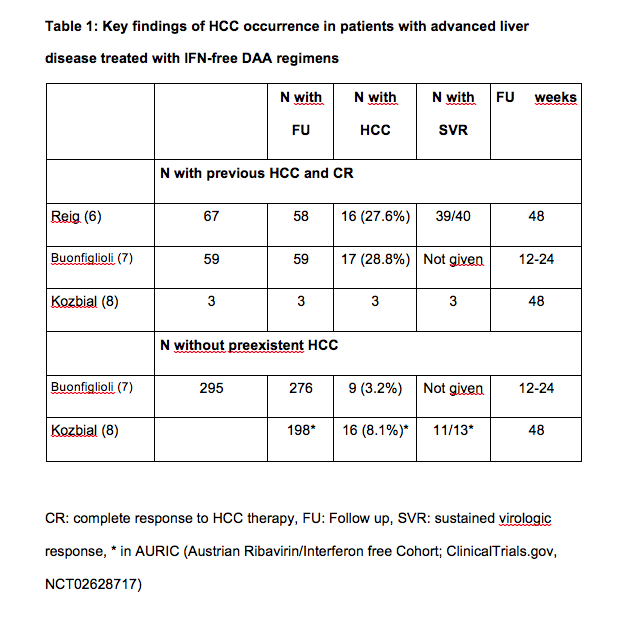
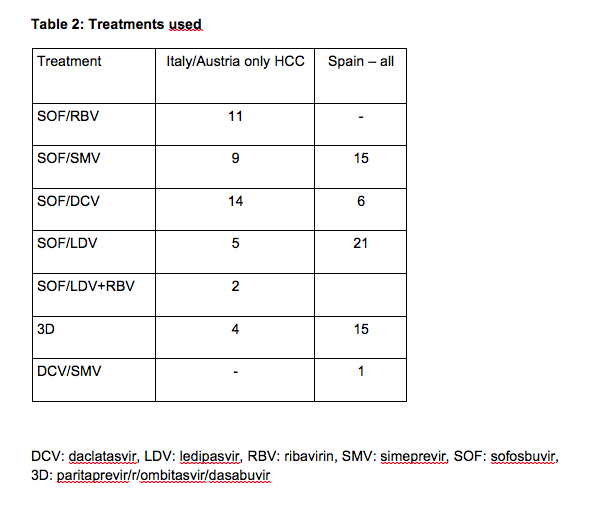
Now, with the availability of highly effective and safe interferon-free combinations even patients with advanced liver disease can be treated successfully. Recently, several groups observed an unexpected large number of HCC occurring in patients with advanced liver diseases within months following successful interferon-free antiviral treatment (see tables 1 and 2). Researchers from Barcelona treated 67 patients with HCV infection and prior history of treated HCC who achieved complete response and lacked ‘non-characterized nodules’ at the start of treatment with all-oral direct acting antivirals(3). Fifty-eight of them completed follow-up. After a median follow-up of 5.7 months, 3 patients died and 16 developed radiologic tumor recurrence (27.6%). In northern Italy, 344 patients with HCV cirrhosis were treated with IFN-free DAA combinations(4). This cohort included 59 patients with previous HCC and 295 without HCC. Among the HCC patients, tumor recurrence was noted in 28.8%, similar to the rate observed in Barcelona. The rate of de novo HCC was substantially lower but still high. In Austria 19 patients were diagnosed with HCC shortly after treatment with DAA. Sixteen patients (11 with SVR) were treated with IFN-free DAA regimens without and 3 (all with SVR) with ribavirin(5). Eleven of the 16 cirrhotic patients without evidence of HCC in their medical history had a SVR 48, 5 had a viral relapse. Three patients with complete remission of pre-existent HCC (surgical resection in one, radiofrequency ablation in 2) experienced tumor recurrence shortly after achieving SVR.
Q. Is treatment with DAA really associated with increased rates of HCC occurrence and recurrence? Is there a risk of biased observations?
There is no clear explanation for this observation, but this high number raises the suspicion of a possible relation of HCC with IFN-free DAA therapy. Obviously, patients with advanced liver disease are at high risk for HCC and possibly HCC occurred just by chance in temporal relation to antiviral therapy. Pretreatment, even the best imaging methods cannot exclude a small HCC with certainty(6).
Furthermore, patients who would not have tolerated IFN-based therapies represent a very high-risk group. A recent paper from Japan reported a two-fold increase in HCC rates in patients treated with IFN-free regimens as compared to dual therapy(7) assuming that higher age and more advanced stage of liver disease as risk factors for HCC in their DAA patients. A further explanation for these unexpected observations are that a major regeneratory stimulus by curing inflammation and the changed immunologic environment compared to treatment with IFN-containing treatments leads to growth of precancerous lesions or of small malignant cell clones(8). Interferon is an immune modulator with antiproliferative properties. With IFN-free treatments, it has been shown that the rapid disappearance of HCV leads to the reconstitution of innate immunity(9) and downregulation of type II and III IFNs, their receptors, and interferon stimulated genes(10,11).The lack of interferon activation may allow growth of malignant cells.
Q. Professor Ferenci, what do you recommend to optimize DAA therapy of patients with advanced HCV?
First, one should try to treat patients as early as possible, before they become cirrhotic. Then we may not have to be worried about HCC occurrence at all.
In patients on the transplant waiting list one should consider treating them after successful transplantation. Otherwise, screening at short intervals will help to diagnose HCC in a very early stage, when treatment options are excellent.
References
- van der Meer AJ, Wedemeyer H, Feld JJ, Dufour JF, Zeuzem S, Hansen BE, Janssen HL, et al. Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA. 2014;312:1927-1928.
- Scherzer TM, Reddy KR, Wrba F, Hofer H, Staufer K, Steindl-Munda P, Gangl A, Ferenci P. Hepatocellular carcinoma in long-term sustained virological responders following antiviral combination therapy for chronic hepatitis C.
J Viral Hepatitis 2008:15: 659–665. - Reig M., Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, et al. Unexpected early tumor recurrence in patients with hepatitis C virus-related hepatocellular carcinoma undergoing interferon-free therapy: a note of caution. J. Hepatol (2016), doi:http://dx.doi.org/10.1016/j.jhep.2016.04.008
- Buonfiglioli F, Conti F, Andreone P, Crespi C, Foschi FG, Lenzi M et al. Development of hepatocellular carcinoma in HCV cirrhotic patients treated with direct acting antivirals. Late Breaker Abstract, 51. Annual Meeting of EASL, 2016.
- Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with SVR following IFN-free DAA treatment. J Hepatol 2016.doi:10.1016/j.jhep.2016.06.009.
- Park MS, Kim S, Patel J, Hajdu CH, Do RK, Mannelli L, et al.
Hepatocellular carcinoma: detection with diffusion-weighted versus contrast-enhanced magnetic resonance imaging in pretransplant patients. Hepatology 2012;56:140-148. - Toyoda H, Kumada T, Tada T. Changes in patient backgrounds may increase the incidence of HCC after SVR in the era of IFN-free therapy for HCV. Hepatology 2016 (e-pub). http//dx.doi.org/10.1022/hep28632
- Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844–855.
- Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, et al. Successful interferon-free therapy of chronic hepatitis C virus Infection normalizes natural killer cell function. Gastroenterology 2015;149:190–200.
- Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124:3352-3363.
- Meissner EG, Kohli A, Virtaneva K, Sturdevant D, Martens C, Porcella SF, et al. Achieving sustained virologic response after interferon-free hepatitis C virus treatment correlates with hepatic interferon gene expression changes independent of cirrhosis. J Viral Hepatitis. 2016 http//dx.doi.org/10.111jvh.12510
Prof. Peter Ferenci M.D.
Division of Gastroenterology and Hepatology
Department of Internal Medicine III
Medical University of Vienna
Waehringer Guertel 18-20
1090 Vienna,
Austria
Email: [email protected]
Katja Deterding M.D.1, Heiner Wedemeyer M.D.2 and Michael P Manns M.D.3
Department of Gastroenterology, Hepatology and Endocrinology
Hannover Medical School, Hannover, Germany
Q. Professor Manns, how frequent is acute hepatitis C nowadays?
Infection with hepatitis C virus (HCV) is one of the main causes of chronic liver disease worldwide, and often leads to end-stage liver disease and hepatocellular carcinoma (HCC). Incidence of acute HCV infections has declined in recent years following the introduction of blood-donor screening in the early 1990s. However, new infections do occur and common risk factors include intravenous drug use(1), medical procedures(2,3) and sexual intercourse(1,4) with people infected with HCV.
Q. Is there any effective treatment and is it worth trying?
Antiviral treatment of acute hepatitis C with interferon alfa is well established. High cure rates have been reported with 12-24 weeks of antiviral treatment(1,5-7) and in contrast to interferon treatment of chronic hepatitis C ribavirin co-administration was not required in acute hepatitis C in most settings. The German Hep-Net Acute HCV-I Study, which was conducted at 24 centres in a real-world setting, assessed 24 weeks of antiviral treatment with standard interferon alfa-2b for patients with acute hepatitis C and reported that 98% of patients who adhered to treatment had a sustained virological response (SVR)(8). The concept of immediate treatment was transferred to pegylated interferon in the German Hep-Net Acute HCV-II study(9). Patients were treated with pegylated interferon alfa-2b 1.5 µg/kg once weekly s.c. for 24 weeks. The sustained virological response rate (SVR) in individuals adherent to therapy was 89%. Timing of treatment has been discussed widely in the past years. Therefore, the aim of the German Hep-Net Acute HCV-III Study was to compare immediate pegylated interferon alfa-2b treatment for six months versus delayed treatment with pegylated interferon alfa-2b plus ribavirin for six months(10). The infection was cured in 90-93% of cases while later therapy was associated with higher lost-to-follow-up rates.
Q. Can interferon-based therapy be replaced by DAA?
Yes, interferon-based therapies can lead to frequent and sometimes severe side effects and many patients cannot be treated with interferon alfa due to contraindications. Several novel direct acting antivirals (DAA) against HCV have been approved in the last two years thus allowing interferon-free therapy of chronic hepatitis C. Overall, more than 95% of patients with chronic hepatitis C can now be cured with new treatment options across different stages of liver disease and HCV genotypes(11). Interferon-free treatment of acute hepatitis C with DAA has not been investigated in depth until now. Preliminary reports on case series or smaller prospective treatment trials exploring IFN-free therapy of acute hepatitis C have been presented during conferences. At AASLD 2015, data from a single centre case series were presented. HIV-infected men with acute hepatitis C received after a 12-week observation period for spontaneous clearance, sofosbuvir plus ribavirin for 12 weeks. SVR-12 rate was 92%(12). A multicentre, single arm trial of the AIDS Clinical trial Group (ACTG) investigating the safety and efficacy of 12 weeks of sofosbuvir and weight-based ribavirin for the treatment of acute HCV infection in HIV-infected participants showed an SVR-12 rate of 90%(13). All HCV genotypes were included in this trial. Neither Il28B genotype nor early, on-treatment viral kinetics were associated with treatment outcome.
Q. Can duration of DAA therapy of acute HCV infection be shortened?
There were trials investigating shorter duration of antiviral treatment of acute hepatitis C. Ledipasvir/sofosbuvir was administered for only six weeks in HIV-infected patients with acute hepatitis C. HCV genotype 1 or 4 were included in this trial. The results showed that antiviral treatment with ledipasvir/sofosbuvir for 6 weeks resulted in an SVR 12 rate of 77%. Three patients with very high viral load at baseline relapsed after the end of antiviral treatment(14). Therefore, HIV-infected patients with acute hepatitis C and a high baseline viral load may be considered for longer duration of therapy. Antiviral treatment was well-tolerated.
Interim data of a clinical pilot study (SLAM C study) from patients with acute HCV mono-infection were presented at AASLD 2015. Twenty-nine patients received sofosbuvir with ledipasvir for 4 weeks or sofosbuvir plus simeprevir for 8 weeks(15). This study demonstrates a high SVR with short-course DAAs in acute hepatitis C with SVR (at weeks 20) >90% in both groups.
The first data of the German HepNet-Acute-HCV-IV study were presented at EASL ILC 2016(16). This study was a prospective, multicentre pilot trial to evaluate the efficacy and safety of ledipasvir plus sofosbuvir (LDV/SOF) for 6 weeks in patients with acute HCV genotype 1 monoinfection. Twenty patients were included from ten centres The main risk factors for HCV infection were sexual transmission and medical procedures/needle stick injuries. At follow-up week 12, 18 patients had normalized ALT and 18 patients had normal bilirubin values. All patients achieved a sustained virological response (SVR-12 20/20 (100%)). Treatment was well-tolerated; there were no drug-related serious adverse events. The findings of the study could have very important implications for clinical practice.
Q. Thus at variance with interferon, DAA should be given early during acute HCV, is this true?
In the absence of a vaccine against HCV, early treatment of acute hepatitis C will likely be needed to prevent the spread of HCV in high-risk populations. Early therapy of acute hepatitis C will also shorten disease and reduce infection-associated morbidity. Medical health-professionals infected after occupational exposure would also benefit from treatment of acute hepatitis C as they could continue to work. Finally, early (shorter) treatment of acute hepatitis C could potentially be cost-saving compared to standard therapy of chronic hepatitis C. In addition, the results show that antiviral treatment with IFN-free regimen rapidly improves symptoms of acute hepatitis.
In conclusion, the high efficacy of 6 weeks of IFN-free treatment of acute hepatitis C needs to be confirmed for other HCV genotypes and other treatment regimens and maybe even shorter treatment durations could be investigated.
References
- Grebely J, Prins M, Hellard M, Cox AL, Osburn WO, Lauer G, et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. The Lancet Infectious Diseases. 2012;12(5):408-14. Epub 2012/05/01.
- Deterding K, Wiegand J, Gruner N, Hahn A, Jackel E, Jung MC, et al. The German Hep-Net acute hepatitis C cohort: impact of viral and host factors on the initial presentation of acute hepatitis C virus infection. Zeitschrift fur Gastroenterologie. 2009;47(6):531-40. Epub 2009/06/18.
- Martinez-Bauer E, Forns X, Armelles M, Planas R, Sola R, Vergara M, et al. Hospital admission is a relevant source of hepatitis C virus acquisition in Spain. Journal of Hepatology. 2008;48(1):20-7. Epub 2007/11/14.
- Terrault NA, Dodge JL, Murphy EL, Tavis JE, Kiss A, Levin TR, et al. Sexual transmission of HCV among monogamous heterosexual couples: The HCV partners study. Hepatology. 2012. Epub 2012/11/24.
- Santantonio T, Fasano M, Sagnelli E, Tundo P, Babudieri S, Fabris P, et al. Acute hepatitis C: a 24-week course of pegylated interferon alpha-2b versus a 12-week course of pegylated interferon alpha-2b alone or with ribavirin. Hepatology. 2014;59(6):2101-9. Epub 2014/01/21.
- Hullegie SJ, Claassen MA, van den Berk GE, van der Meer JT, Posthouwer D, Lauw FN, et al. Boceprevir, peginterferon and ribavirin for acute hepatitis C in HIV infected patients. Journal of Hepatology. 2016;64(4):807-12. Epub 2015/12/23.
- Doyle JS, Deterding K, Grebely J, Wedemeyer H, Sacks-Davis R, Spelman T, et al. Response to treatment following recently acquired hepatitis C virus infection in a multicentre collaborative cohort. Journal of Viral Hepatitis. 2015;22(12):1020-32. Epub 2015/06/23.
- Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. The New England Journal of Medicine. 2001;345(20):1452-7. Epub 2002/01/17.
- Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250-6. Epub 2006/01/28.
- Deterding K, Gruner N, Buggisch P, Wiegand J, Galle PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. The Lancet Infectious Diseases. 2013;13(6):497-506. Epub 2013/03/26.
- Wedemeyer H. Towards interferon-free treatment for all HCV genotypes. Lancet. 2015;385(9986):2443-5. Epub 2015/04/04.
- Fierer DSB, Z.; Dieterich, D.; Foster, A.L.; Morey, T.; Turner, S. Sofosbuvir in the Treatment of Acute HCV Infection in HIV-infected Men. Hepatology. 2015;62:Abstract 1090.
- Naggie SM, K.M.; Hughes, M.; Fierer, D.S.; Kim, A.Y.; Hollabaugh, K.; Kiser, J.; Roa, J.; Symonds, B.; Brainard, D.M.; McHutchinson, J.G.; Peters, M.G.; Chung, R.T.;. Sofosbuvir Plus Ribavirin Without Interferon For Treatment of Acute Heatitis C Virus Infection in HIV-1 infected Individuals (SWIFT-C). Hepatology. 2015;62:Abstract 1094.
- Rockstroh JKB, S.; Hyland, R.H.; Yun, C.; Zheng, W.; Brainard, D.M.; McHutchinson, J.G.; Ingiliz, P.; Lutz, T.; Nelson, M. Ledipasvir/Sofosbuvir for 6 weeks in HIV-Infected Patients with Acute HCV Infection. CROI. 2016.
- Basu PSNJJ, N.; Aloysius, M.; Brown, R. Sofosbuvir and Ledipasvir versus Sofosbuvir and Simeprevir combination therapy in the management of acute hepatitis C: A randomized open label prospective clinical pilot study. SLAM C study. Interim data. Hepatology. 2015;62:Abstract 1074.
- Deterding KS, C.;Schott, E.;Welzel, T.;Gerken, G.;Klinker, H.;Spengler, U.;Wiegand, J.;Schulze zur Wiesch, J.;Pathil, A;Cornberg, M.;Umgelter, A.;Zöllner, C.;Zeuzem, S.;von der Leyen, H.;von Witzendorff, D.;Manns, M.P.;Wedemeyer, H. Six weeks of sofosbuvir/ledipasvir (SOF/LDV) are sufficient to treat acute Hepatitis C virus genotype 1 monoinfection: The HepNet acute HCV IV study. Journal of Hepatology. 2016;64:S183-S212, Abstract LB08, EASL 2016
Prof. Michael P. Manns, M.D.
Professor and Chairman
Department of Gastroenterology, Hepatology and Endocrinology
Hannover Medical School
Carl-Neuberg- Strasse 1
D-30625 Hannover, Germany
Email: [email protected]
Robert G. Gish, M.D.1,2,3,4,5 and Doan Y Dao, M.D.4,6,7
1 Department of Medicine, Division of Gastroenterology and Hepatology, Stanford University, Stanford, California, USA
2 Hepatitis B Foundation, Doylestown, Pennsylvania, USA
3 National Viral Hepatitis Roundtable, San Francisco, California, USA
4 The National Task Force on Hepatitis B, Pikesville, Maryland, USA
5 The FAIR Foundation, Palm Springs, California, USA
6 Center for the Genetics of Host Defense, University of Texas Southwestern Medical Center, Dallas, Texas, USA
7 Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Q. There’s a lot of talk nowadays about new approaches for treating chronic hepatitis B infection (CHB). Do you think there is a change in thinking about the overall approach to CHB?
Yes, after more than two decades of being limited to mostly non-curative suppression of viral replication that generally required long-term continuation of therapy to maintain benefits there is now increasing optimism that by combining some of the exciting new therapeutic approaches that are currently being studied we might actually be moving toward some form of cure. Although a true “sterilizing” cure with complete elimination of all viral DNA from the body, including removal of all cccDNA and integrated virus, may be much more difficult, we are much more optimistic about achieving a “functional” cure in which there are virologic, biochemical and histologic responses as well as seroclearance of hepatitis B surface antigen (HBsAg), all of which are sustained off therapy.
There have been considerable recent advances in our understanding of both HBV virology and the host response to it that have led the way to the development of multiple new therapies that are now being studied, including both new direct-acting antivirals (DAAs) and host-targeting antivirals, including both immune modulators and agents that target host function(1). In addition to the possibility of advances in viral control that might come with the use of these as sole agents, there is increasing interest in combinations that might yield optimal therapeutic outcomes(2).
Q. Of the immune modulators now in clinical trials, which do you think are of particular interest?
In terms of immune modulators, the farthest along in development are Toll-like receptor agonists, immune checkpoint inhibitors, therapeutic vaccines, and engineered T cells. Each of these has shown some promise in early work although much more research will be required to understand which will have the most value clinically and which will be practical for worldwide use. Ultimately, the best results are most likely to come with some combination of such immune modulators with DAAs. Because the efficacy of some immune modulators, including therapeutic vaccines, checkpoint inhibitors, and interferon, is known to be compromised by high antigen levels(3-5) there is interest in combining these agents with approaches to reducing viral antigen levels.
As one example, RNA interference (RNAi) has been shown to lower HBsAg via elimination of HBV RNA transcripts(6). In a recent study, the combination of entecavir and ARC-520, the RNAi therapy that is currently the farthest along in development, yielded a substantial and prolonged reduction in viral antigens including HBsAg. This type of substantial reduction could be expected to improve the response to a number of the immune modulators.
Q. Has awareness of the public health burden related to CHB improved in the U.S. and do current policies and attitudes support identifying CHB patients? Advances in therapeutic approaches to CHB won’t accomplish anything unless people with chronic infection are identified.
During recent years we have been very pleased that both major institutions‒including the Institute of Medicine and the Division of Viral Hepatitis of the U.S. Centers for Disease Control and Prevention (CDC)‒and respected U.S. researchers have published reports based on sound scientific evidence that recognize the substantial viral hepatitis public health burden in the U.S. and the urgent need to address it, and have proposed strategic plans for accomplishing this(7,8). Several recent studies have supported our long-time belief that the prevalence of CHB in the U.S. is actually substantially higher than was previously thought(9,10). We think that the stories in the national press that these studies generated improved public awareness.
There has also been an important policy change that has the potential to substantially increase screening for HBV infection. In 2014, the US Preventive Services Task Force (U.S.P.S.T.F.), an independent panel of experts in primary care and prevention who review and analyze the cost-effectiveness of health services, changed its recommendation on HBV screening from category D “the U.S.P.S.T.F. recommends against the service” with advice to health care providers to “discourage the use of this service” to category B “the U.S.P.S.T.F. recommends the service” with advice to health care providers to “offer or provide this service to at risk groups(11)”. The at risk populations are defined as foreign-born Americans from regions where the prevalence of CHB is at least 2% (essentially all countries in sub-Saharan Africa and Asia and some in the South America-Amazon basin area), injection drug users, men who have sex with men (MSM), and renal dialysis patients. This is an important development related to treatment since screening is the first step toward identifying patients with CHB and then referring them for care. Studies have shown that a provider’s recommendation is one of the chief determinants of whether testing for viral hepatitis occurs(12). Moreover, this updated recommendation from the U.S.P.S.T.F. has enhanced the voice of U.S. hepatitis B advocacy groups at all levels.
References
- Block TM, Rawat S, Brosgart CL. Chronic hepatitis B: A wave of new therapies on the horizon. Antiviral Res 2015;12169-81.
- Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, Schluep T. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res 2015;12147-58.
- Barnes E. Therapeutic vaccines in HBV: lessons from HCV. Med Microbiol Immunol 2015;204(1):79-86.
- Weng M, Zeng WZ, Wu XL, Zhang Y, Jiang MD, Wang Z, Zhou DJ, et al. Quantification of serum hepatitis B surface antigen in predicting the response of pegylated interferon alfa-2a in HBeAg-positive chronic hepatitis B with prior lamivudine exposure. Virol J 2013;10277.
- Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med 2002;195(9):1089-1101.
- Gish RG, Yuen MF, Chan HL, Given BD, Lai CL, Locarnini SA, Lau JY, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res 2015;12197-108.
- Gish RG, Cohen CA, Block JM, Brosgart CL, Block TM, Clary R, Le LT, et al. Data supporting updating estimates of the prevalence of chronic hepatitis B and C in the United States. Hepatology 2015;62(5):1339-1341.
- Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. The National Academies Press, Washington, D. C., 2010.
- Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 2012;56(2):422-433.
- Cohen C, Evans AA, London WT, Block J, Conti M, Block T. Underestimation of chronic hepatitis B virus infection in the United States of America. J Viral Hepat 2008;15(1):12-13.
- LeFevre ML, Force USPST. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161(1):58-66.
- Nguyen TT, McPhee SJ, Stewart S, Gildengorin G, Zhang L, Wong C, Maxwell AE, et al. Factors associated with hepatitis B testing among Vietnamese Americans. J Gen Intern Med 2010;25(7):694-700.
Dr. Robert G.Gish M.D.
Department of Medicine,
Division of Gastroenterology and Hepatology,
Stanford University, Stanford, California, USA
Email: [email protected]
Dr. Antonio Boschini,
Medical Director of the San Patrignano Rehabilitation Community, Italy
Q. Dr. Boschini, how many PWIDs are resident in the Community?
At the moment, our Community is host to about 1228 residents, all of whom are in recovery for addiction to illegal substances (DU), of these, 681 (55,5%) have a past of intravenous drug use. (PWID)
Q. How long do the residents stay for rehabilitation and how frequently are they found to be chronically infected by HBV and HCV?
Our therapeutic program lasts four years. This is a residential therapeutic program, which is based essentially on education, rehabilitation and professional training for reintegration. We do not use opioid replacement therapy. We only prescribe psychopharmacological treatments for residents with a recognized psychiatric condition as well as a drug dependency. This applies to 23% of residents. There is a need for individual psychotherapy for approximately 30% of our residents, not for the treatment of addiction itself but problems that are associated to addiction, such as sexual abuse, PTSD, eating disorders and so forth.
The prevalence of HCV, HBV, and HIV infections in the people that are undergoing treatment is 34%, 0,6% and 3,2% respectively whereas among PWIDs the prevalence is 59,4%, 0,7% and 7,2% respectively.
Q. What is the standard of care in terms of life style and surveillance and therapy of viral hepatitis?
The Community has a dedicated medical center that comprises a health center, two hospital wards with a total of 50 beds for patients with serious diseases or illnesses related to drug use (AIDS, cirrhosis, cancer, neurological diseases, tuberculosis, etc.), a laboratory for blood and urine analysis (with a serum repository with frozen samples of more than 20,000 individuals), physiotherapy, etc. On admission to the Community, every guest undergoes a medical screening that includes: (a) toxicological and clinical medical history; (b) medical examination; (c) chest x ray; (d) PPD skin test; (e) ECG; (f) spirometry; (g) blood chemistry analysis which includes, tests for HIV, HBV, HCV, and syphilis. Those who result HCV or HBV positive undergo an ECO scan and complete virological analysis.
Until 2013 the standard of care for eligible patients with chronic HCV infection was pegylated interferon plus ribavirin, but most of them suffered neuropsychiatric side effects, a side effect more frequent in former drug users than in other populations. Now interferon based therapy is prescribed only to non-cirrhotic genotype 3 infected patients with a negative anamnesis for psychiatric diseases.
Q. How many residents are being treated with IFN-free DAAs?
To date, twenty patients, all with cirrhosis or HCC have been treated with IFN-free regimens.
Q. Is there a Scientific Committee to supervise management of residents’ medical and social problems?
The Scientific Committee was founded in 1984. It oversees the medical needs of the Community’s residents and evaluates both the ongoing scientific research projects and those that the Community intends to pursue.
Q. What initiatives has the Scientific Committee undertaken for 2017?
We are initiating screening for those residents with HCV and HBV using FibroScan with the aim of identifying HCV infected patients who are entitled to receive DAAs according to AIFA criteria. For 2017 our goal is to treat as many patients as possible, absolutely all of those co-infected with HCV and HIV and HCV and HBV, those with fibrosis> 3, and possibly even some patients with a less advanced fibrosis.
Dr. Antonio Boschini M.D.
Medical Director
San Patrignano Rehabilitation Community
Via San Patrignano, 53
47853 Coriano – Rimini – Italy
Email: [email protected]
Dr. Laurent Castera,
EASL Secretary General
Q. Dr. Castera, can you sum up briefly the 2016 International Liver Congress?
The 51th Annual Meeting of the European Association for the Study of Liver (EASL), the so-called International Liver Congress™ (ILC), was held this year in Barcelona, Spain 13-17 April. It was a great success with over 10,000 healthcare professionals and almost 1,000 young investigators from 120 countries in attendance. A record number of abstracts, nearly 3000, were received, of which more than 150 were selected as oral presentations and late breakers, providing the attendees with cutting-edge science in liver research from around the world. This year, the honorary President of the ILC was Daniel Shouval, Professor at the University of Jerusalem, Israel.
Q. What was the Congress’s approach to education in liver diseases?
The highlight of the educational program was, without doubt, the post-graduate course dedicated to the management of liver tumors, including hepatocellular carcinoma, intrahepatic cholangiocarcinoma and benign liver tumors, an area of hepatology that has significantly advanced over the past decade. In parallel, there was the basic science seminar targeted at researchers and clinicians interested in translational medicine. This seminar provided an overview of the advances in the growing field of the gut-liver axis. It featured interactive sessions dealing with gut microbiota and immunity, microbiota and metabolism, microbiota and liver disease as well as diagnostic and therapeutic strategies.
Q. Which presentations dealt with research and clinical advancements?
The two State-of-the-Art lectures feature among the highlights of this year’s congress. The clinical lecture, given by Professor Fabien Zoulim, from Lyon University, France on “New treatment paradigm to cure chronic hepatitis B virus infections” and the basic one, by Michael Karin, Distinguished Professor of Pharmacology and Pathology at the University of California in San Diego, USA, on “Mechanisms responsible for malignant conversion”. Both lectures were highly appreciated.
Interestingly, several new initiatives were launched successfully this year and represent highlights of the ILC in Barcelona: (1) the “Fusion Hepatology” session, dedicated to the differences in presentation and management of NAFLD between Asian (Korea and Japan) and western countries (France and USA); (2) the best poster session, summarizing the best research presented as posters in in the fields of clinical and basic hepatology and the poster tours, with key opinion leaders guiding the audience through a selection of posters; (3) Symposia highlighting the newly published EASL Clinical Practice Guidelines (CPG), including those on NAFLD with the European Association for the Study of Diabetes (EASD) and the European Association for the Obesity (EASO), CPGs on autoimmune hepatitis, liver transplantation, benign liver tumors, and vascular liver disease and finally, an update on treatment of hepatitis C to be published in September; (4) Joint symposia with other European medical organizations: NAFLD with EASD, hepatocellular carcinoma with the European Society for Gastrointestinal and Abdominal Radiology (ESGAR), liver elastography with the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB), and a post-graduate teaching in Liver Transplantation with the International Liver Transplantation Society (ILTS), the European Liver and Intestine Transplant Association (ELITA), and the Liver Intensive Care Group of Europe (LICAGE).
Q. Were there any breakthroughs presented in therapy of liver disease with respect to the ILC 2015?
As for the original scientific communications, once again, viral hepatitis was one of the main themes with several clinical and basic parallel sessions on viral hepatitis B, C and D throughout the meeting. Presentations on HCV included real-life data on the use of direct acting antivirals (DAA) in large cirrhotic populations as well as trials on the use of DAA in special populations (patients with chronic kidney disease, kidney transplant, and decompensated cirrhosis), reduction of DAA treatment duration (8 weeks) and re-treatment of non-responders to current DAA. In addition, data showing high efficacy of generics DAA were presented during the late breaker session. As for HBV, two phase-III studies reported on similar efficacy with less renal toxicity of tenofovir alafenamide as compared with tenofovir disoproxil fumarate in both HBe Ag positive and negative patients as well as preliminary data on potential novel therapeutic strategies such as combination of an HBV core inhibitor with peginterferon. Apart from viral hepatitis, many interesting data were also presented including novel molecular classification of hepatocellular adenoma, use of norursodeoxycholic acid in primary sclerosing cholangitis, novel therapeutic approaches using FXR agonists in NAFLD, use of alfapump system for the treatment of refractory ascites and validation of the new non-invasive Baveno VI criteria for screening eosophageal varices in patients with cirrhosis.
Q. How many hepatologists were awarded for their contributions in the field?
The EASL Recognition Awards ceremony was another highlight of the meeting. Three prominent members of our community, two Europeans and one American, were awarded for their outstanding contribution to the field of liver diseases. Prof. Antonio Craxì, University of Palermo, Palermo, Italy for his work in viral hepatitis, Prof. Jordi Bruix, University of Barcelona, Barcelona, Spain for his research on hepatocellular carcinoma and Prof. Roberto J. Groszmann, Yale University, New Haven, Connecticut, USA for his work in cirrhosis and portal hypertension. In addition, for the second year running, two young investigators researchers received the EASL Young Investigator Award: Jordi Gracia-Sancho, University of Barcelona, Barcelona, Spain and Veronica Lukacs-Korneck, University of Saarland, Homburg, Germany.
Q. Dr. Castera, what’s next?
Well, we look forward to seeing you at next year’s International Liver Congress™in Amsterdam, The Netherlands 19-23 April!
Dr. Laurent Castera M.D., PhD
Department of Hepatology
Hôpital Beaujon, AP-HP
INSERM U773
University of Paris-VII
92110 Clichy, France
Email: [email protected]
Web: www.easl.eu

