Contents
by Prof. Dr Ralf Bartenschlager, Asian Oncology Summit (AOS) 2015
University of Heidelberg and German Cancer Research Centre, Heidelberg, Germany
Persistent infections with the hepatitis C virus (HCV) are a leading cause of chronic liver disease. It is estimated that around 130 million people worldwide are persistently infected with this virus and these people are at high risk to develop hepatosteatosis, liver cirrhosis and eventually hepatocellular carcinoma. Although with the implementation of diagnostic tests to exclude HBV-containing blood products the number of transfusion-associated hepatitis cases had declined dramatically, it became clear that an additional blood-transmitted virus exists. The search for this pathogen that by exclusion criteria was initially called non-A, non-B hepatitis virus, was a major challenge because of the unknown nature of the virus, its low amounts in infected tissue and the limited sensitivity of available techniques to isolate an unknown agent. It is therefore little surprising that many research groups from academia and industry failed to discover the virus, but with the advent of an immune-screening approach, the group of Michael Houghton and his team at Chiron Corporation in Emeryville, California finally succeeded in isolating the genome of an RNA virus, called HCV. Through a collaborative effort they established a diagnostic test demonstrating that people with post-transfusion non-A, non-B hepatitis indeed have antibodies against antigens derived from the cloned HCV genome. This discovery was the starting point for the development of antiviral drugs to treat chronic hepatitis C that included four tasks to which industry and academia contributed to various extents:
First, to unravel the organization of the HCV genome. This was mainly driven by academic research groups that identified polyprotein cleavage products and mechanisms, involved viral and cellular enzymes as well as RNA elements required for RNA translation and replication.
Second, to identify and characterize the main viral targets for antiviral therapy. There, both academia and industry contributed substantially by determining the 3D X-ray crystal structures of the NS3-4A protease complex and the NS5B RNA-dependent RNA polymerase, which are two of the three main viral drug targets. In addition, in-depth biochemical analyses of the protease revealed the requirement for a viral co-factor and end product inhibition, which laid the ground for first-generation protease inhibitors. In addition, the biochemical properties of the NS5B polymerase were discovered revealing a de novo priming mechanism as well as a peculiar allosteric GTP binding site.
Third, to establish cell culture models supporting robust and easy to measure HCV replication in cultured cell lines. This task that was mainly driven by academic groups which turned out to be very challenging because for reasons we still do not understand completely, even HCV isolates with proven infectivity in vivo are unable to replicate efficiently in cultured cell lines. An important work-around was thus the establishment of the subgenomic replicon system that is based on genetically engineered HCV “mini-genomes” that comprise just the replicase factors of HCV (which comprise NS3 and NS5B) and the cis-acting RNA elements of the viral genome. First-generation replicons were capable of efficient self-replication in the human hepatoma cell line Huh7 and provided the first cell culture system for validation of antiviral drugs that had been developed with (surrogate) in vitro systems. In addition, the replicon system that recapitulated all the intracellular steps of the HCV replication cycle lead to the discovery of important host cell factors that turned out to be promising antiviral drug targets (e.g. cyclophilin A or microRNA-122). Moreover, replicons were used extensively for random screening approaches of drug libraries, ultimately leading to the discovery of highly potent inhibitors of NS5A. This result was remarkable because NS5A lacks enzymatic activity and thus, had not been considered as a drug target. Apart from that, the replicon system paved the way for the subsequent establishment of a fully permissive HCV cell culture system that became possible with the molecular cloning of the very unusual HCV isolate JFH-1 that had been a discovery by Dr. Takaji Wakita at the University of Tokyo. This isolate replicates efficiently in cell lines and is infectious in vivo and closed the “gaps” of the replicon system: the study of the early and late steps of the HCV replication cycle (binding to and entry into the host cell and assembly as well as release of infectious virus particles, respectively).
The fourth task was to develop HCV-specific antiviral drugs, which was mainly driven by industry. These activities lead to the development of highly efficient inhibitors targeting the NS5B polymerase, NS5A and the NS3 protease. Inhibitors of the first-generation had some bias towards HCV genotype 1b, because the first available replicon was based on an HCV isolate of this genotype. However, with the establishment of replicons of other genotypes and intergenotypic HCV chimeras, it became possible to optimize the drugs towards broader genotype coverage. All these activities finally lead to the establishment of interferon-free anti-HCV treatment that is highly successful, allowing virus eradication in around 90% of treated patients.
Taken together the implementation of antiviral therapy to treat chronic hepatitis C is the result of research and development activities conducted by groups in academia and industry, contributing to the different tasks to various extents and according to their main “mission”. In retrospect, it took about 25 years from the discovery of HCV until a cure for most patients was developed. Probably the main turning point was the implementation of cell culture systems for HCV enabling random drug screening as well as compound validation. In the case of HIV it took only a few years from the discovery of the virus that replicates reasonably well in cell culture, until the first antiviral drugs became available. The longer time it took in the case of HCV reflects, at least in part, the time it took from the discovery of the virus until a system was found to propagate it in cell culture.
Prof. Dr. Ralf Bartenschlager
University of Heidelberg
Department of Infectious Diseases
Molecular Virology
INF 345, 1st. Floor
D-69120 Heidelberg
Germany
Email: [email protected]
by Samuel W. Brayer, BS and K. Rajender Reddy, MD University of Pennsylvania USA.
The newly available, all-oral, regimens of hepatitis C virus therapies consist of combinations of potent direct acting anti-viral (DAA) agents with or without ribavirin. In clinical trials, these treatments were safe, well tolerated, and achieved high rates of sustained virologic response 12 weeks after cessation of therapy (SVR12). However, outcomes in heterogeneous real-world populations typically fail to match those in clinical trials which have strict inclusion/exclusion criteria. Thus, the success of these promising regimens in the real world remains to be seen. To that end, two experiences, predominantly in the US, are collecting real-life clinical data on the safety, tolerability, and efficacy of all-oral combinations in patients treated at academic and community medical centers. Results from these trials were presented at the recent EASL 2015 International Liver Congress in Vienna, Austria.
HCV-TARGET is an international consortium of academic and community medical centers in the United States, Germany, Israel, and Canada conducting a longitudinal, observational study to investigate the effects of DAAs in the clinical setting. Results from an analysis of patients with decompensated cirrhosis were presented at the recent EASL meeting. For this series, HCV patients aged 18 years and older were enrolled if they had been treated with an all-oral DAA regimen according to standard of care at the local study sites. In order to be included in this analysis, patients were required to have a baseline model for end-stage liver disease (MELD) score greater than or equal to 10. The primary aim of the analysis was to evaluate SVR12 while safety was assessed as well. Between December, 2013 and October, 2014 2,204 patients were enrolled. Of those enrolled, 253 had MELD≥10, and at the time of reporting 216 has completed the requisite 12 week follow up period. Patients were treated with sofosbuvir/ribavirin (76 patients), sofosbuvir/simeprevir (108 patients) or sofosbuvir/simeprevir/ribavirin (32 patients). The population was 67.2% male, with a mean age of 59.2 years (range: 38-80), and a various genotypes (GT1: 183 patients, GT2: 30 patients, GT3: 33 patients). 9.1% of patients previously failed a protease inhibitor based therapy. GT1 patients exhibited higher rates of SVR when treated with sofosbuvir/simeprevir than with sofosbuvir/ribavirin (74% vs. 52%). The addition of ribavirin to the sofosbuvir/simeprevir regimen had no effect on outcome. GT1b patients had higher rates of SVR than did GT1a patients. GT2 patients achieved high rates of SVR (81%) when treated with sofosbuvir/ribavirin, while GT3 patients showed a more modest response (39%). Patients with MELD score of 16-21 responded similarly to patients with MELD 10-15 when treated with all-oral therapies. Relapse rates were low for GT1 (26%) and GT2 (7%) patients, but remained high in GT3 patients (46%). There was also an effect of treatment on the severity of liver disease reflected by modest improvements in MELD score, and serum bilirubin and albumin levels. However, SVR was not always associated with such clinical improvements. A multivariate analysis revealed that pre-treatment albumin levels were a strong predictor of achieving SVR12 (OR: 2.52, 95%CI: 1.12-5.71, p=0.026), while elevated bilirubin levels were a significant negative predictor of response (OR: 0.46, 95%CI: 0.28-0.76, p=0.002). GT1a patients trended towards being less likely to achieve SVR12 (OR: 0.39, 95%CI: 0.14-1.07, p=0.069). Of note, while elevated bilirubin was associated with a negative outcome, patients’ baseline MELD scores were not a significant predictor of response. Adverse events (AEs) were common, but generally mild. In total, 202 patients experienced an AE: 78/88(88.64%) patients treated with sofosbuvir/ribavirin, 95/114(83.33%) patients treated with sofosbuvir/simeprevir, and 29/32(90.63%) patients treated with sofosbuvir/simeprevir/ribavirin. Anemia occurred in 28/88(31.82%) of patients in the sofosbuvir/ribavirin group, more frequently than in the other two treatment groups. Photosensitivity was exclusively reported by those on a simeprevir based regimen. A total of 44 patients experienced a serious adverse event while on treatment. Events of hepatic decompensation occurred in 16 total patients, and 3 patients died while on treatment. In the sofosbuvir/simeprevir group, one patient died of hepatic failure, and another died due to shock. In the sofosbuvir/simeprevir/ribavirin group, one patient passed away due to unspecified causes. 12 total patients (5.1%) underwent liver transplantation during their course of therapy. Long-term follow up will help determine what baseline characteristics and on-treatment events are predictors of treatment success, and what the true benefits of SVR are on the severity of liver disease. The treatment of GT3 patients remains a large unmet need.
In an additional HCV TARGET EASL presentation, the efficacy of sofosbuvir-containing regimens in GT4 patients (N=75) was assessed. Preliminary safety results indicate that pegylated-interferon/ribavirin/sofosbuvir, sofosbuvir/ribavirin, sofosbuvir/simeprevir, and sofosbuvir/simeprevir/ribavirin are generally safe and well tolerated across a broad spectrum of patients. Among patients for whom post-treatment data is available, 14/16(88%) and 4/4(100%) of GT4 patients treated with pegylated-interferon/sofosbuvir/ribavirin and sofosbuvir/simeprevir+/-ribavirin, respectively, achieved SVR4.
Trio health is a disease management company working with academic medical centers, community hospitals/physicians, and specialty pharmacies. Three analyses were presented examining the outcomes of all-oral combinations of sofosbuvir+/-simeprevir in real-world patient populations at academic and community treatment centers. In all analyses, data were collected from Rx records through the Trio Platform in partnership with AcariaHealth, AllCare Plus Pharmacy, Aureus Health Services and other specialty pharmacies.
A final assessment of 955 (intent to treat [ITT] N=954) patients initiating a 12 week sofosbuvir+/-simeprevir course of therapy between December 2013 and March 2014 at 112 community based and 30 academic medical centers was presented. Patients were treated with pegylated-interferon/ribavirin/sofosbuvir (N=384), ribavirin/sofosbuvir (N=226), or sofosbuvir/simeprevir+/-ribavirin (N=320). 93 patients were excluded from analysis (9 deaths, 38 lost to follow up, 1 transplanted, 45 discontinued) leaving 861 patients in the analysis. Overall, this real-world heterogeneous population achieved high response rates: the SVR12 of the ITT group was 762/954(80%) and 762/861(89%) in the per protocol (PP) patients. Sub-analyses revealed SVR differences by HCV GT (1 vs. 2), subtype (GT1a vs. GT1b), previous treatment status, and the severity of liver disease (cirrhotic vs. non-cirrhotic). Discontinuation rates were low (<5%), and were attributed to AEs, non-adherence, and financial reasons.
An analysis of older patients (aged greater than 70 years) sought to examine the efficacy and safety of pegylated-interferon/ribavirin/sofosbuvir, ribavirin/sofosbuvir, and sofosbuvir/simeprevir+/-ribavirin in a population typically excluded from clinical trials. The patient pool was the same as described in the final assessment above. Of the 954 patients in the study population, the ITT population of patients >70 years was 55 (the remaining 899 patients served as a reference group). Of the 55 patients >70 years, 3 died, 1 was lost to follow up, and 4 discontinued, leaving a PP population of 47 (PP group for patients ≤70 years old, N=814). The >70 year population was 55% male, 10% black, with an average age of 74 years (range: 71-86). Several genotypes and subtypes were represented: GT1 N=1(2%); GT1a N=12(24%); GT2 N=20(36%); GT3 N=18(33%); GT4-6 N=1(2%); Mixed GT N=1(2%). Across all treatments, SVR rates for patients >70 years were numerically different from those younger than 70, though the values did not differ significantly. SVR for the ITT population >70 years was 78% (vs. 80% for ≤70 years) and the PP SVR was 91% (vs. 99 for ≤70 years). In a matched sample SVR analysis of the ITT groups, the SVR rate for patients ≤70 years was significantly higher than for patients >70 years (94% vs. 80%, respectively; P=0.038), however this difference was lost when PP groups were compared (98% vs. 93%, respectively; P=0.275). Adverse events were rare, occurring in only 1 GT1 patient >70 years in the simeprevir/sofosbuvir+/-ribavirin treatment group. In addition to 1 patient each in the GT1 pegylated-interferon/ribavirin/sofosbuvir group and GT2 sofosbuvir/ribavirin group, these 3 patients accounted for all discontinuations in the >70 years old group.
As shown in clinical trials, and replicated in the HCV TARGET study, GT3 is emerging as a difficult to treat population largely due to high relapse rates. A sub-analysis of the Trio cohort examining the use of sofosbuvir/ribavirin for 24 weeks in patients with GT3 infection (N=96 patients, 56% male, 30% with cirrhosis, 39% previously treated) found a 70% SVR12 rate in the ITT population (67/96) and 87% in the PP population (67/77). There was a significant difference between treatment naïve and treatment experienced patients in the PP group (96% vs, 74%; P=0.016). Numerical differences were observed between SVR rates based on practice type (community vs. academic), age (<65 vs. 65+), sex, initial viral load (≤6 million IU/ml vs. >6 million IU/ml), and severity of liver disease (cirrhotic vs. non-cirrhotic).
The results of these large-scale real-life initiatives are reassuring. They corroborate findings from tightly regulated clinical trials, indicating that all-oral therapies are safe, well tolerated, and effective in the general population. Further, they show that patients with decompensated cirrhosis, and older patients can be successfully managed using the available all-oral combinations of DAAs +/- ribavirin. Several interesting aspects of clinical practice have also been elucidated. Of note, while simeprevir is not recommended in patients with advanced liver disease, the HCV TARGET study showed that in practice, it was liberally used to treat this patient population. Simeprevir is an inhibitor of bile transporters (OATP1B1/3) and thus is known to cause mild unconjugated hyperbilirubinemia even in well-compensated liver disease, leading to concerns over its use in patients with advanced disease. However, the safety profiles of these patients are favorable. The treatment of GT3 patients was addressed in both HCV TARGET advanced liver disease patients and in the Trio cohort. While GT3 patients with advanced liver disease remain a clinically challenging population to manage, the Trio data indicate that high SVR rates can be achieved using 24 weeks of sofosbuvir/ribavirin with cirrhosis having no effect and matching SVR rates from the VALENCE clinical trial and thereby validating AASLD guidelines with real-world data. The AASLD guidelines for the treatment of GT4 infection recommend pegylated-interferon/ribavirin/sofosbuvir as an alternative regimen for treatment-naïve patients. Future data from the real world on the use of sofosbuvir-containing regimens in GT4 patients will allow an assessment of the necessity of pegylated-interferon in clinical practice.
K.Rajender Reddy M.D
Ruimy Family President’s Distinguished Professor of Medicine
Professor of Medicine in Surgery
Director of Hepatology
Director, Viral Hepatitis Center
Medical Director, Liver Transplantation
University of Pennsylvania
2 Dulles, Liver Transplant Office
3400 Spruce Street, Philadelphia, PA 19104Email: [email protected]
by Dr. Grace Lai-Hung Wong MBChB (Hons, CUHK), MD (CUHK), FRCP(Edin), FHKCP, FHKAM (Medicine)
Associate Professor, Institute of Digestive Disease, Center of Liver Health, Department of Medicine and Therapeutics, The Chinese University of Hong Kong
 Chronic hepatitis B (CHB)-related hepatocellular carcinoma (HCC) is a major health problem in some European and in most Asian-Pacific regions. There has been unequivocal evidence showing that antiviral therapy reduces the risk of HCC. Nonetheless, the risk has not been eliminated, which is particularly true in those whose serum hepatitis B virus (HBV) DNA has not been completely suppressed.
Chronic hepatitis B (CHB)-related hepatocellular carcinoma (HCC) is a major health problem in some European and in most Asian-Pacific regions. There has been unequivocal evidence showing that antiviral therapy reduces the risk of HCC. Nonetheless, the risk has not been eliminated, which is particularly true in those whose serum hepatitis B virus (HBV) DNA has not been completely suppressed.
It would represent a heavy financial burden for most of the low and middle economic countries to offer antiviral therapy and 6-montly HCC surveillance to all their CHB patients. Therefore, there is a need for accurate and applicable HCC risk prediction to assist prognostication and make decisions on the need for antiviral therapy and HCC surveillance.
A few well-established risk factors for HCC, namely advanced age, male gender, high viral load, cirrhosis etc., are the core components of three well-validated HCC risk scores: CU-HCC, GAG-HCC and REACH-B scores. These 3 scores were confirmed to be accurate in predicting HCC up to 10 years in patients who were mostly treatment-naïve. Nonetheless, in the era of antiviral therapy, patients at risk would have been receiving antiviral therapy. Therefore, it would be more clinically relevant if these scores remain accurate in the presence of antiviral therapy.
The validity and applicability of these three scores have been illustrated recently in our large cohort of Hong Kong patients receiving one of the potent oral antiviral agents, entecavir. We did not just demonstrate the accuracy of these scores before and during entecavir treatment, we also established that decrease in risk scores after antiviral therapy would be translated into a lower risk of HCC.
The Achilles tendon of any risk score that puts heavy weight on presence of cirrhosis (e.g. CU-HCC and GAG-HCC scores) is the incorrect diagnosis of cirrhosis in some cases just based on clinical or radiological parameters. It is well known that early cirrhosis would be easily missed by ultrasonography (USG). With this background, we recently optimized CU-HCC score with liver stiffness measure (LSM) by transient elastography. This new LSM-HCC score provides a highly sensitive prediction to the extent that it excludes future HCC with high negative predictive value (99.4%-100%) at 5years.
All these recent findings support the application of HCC risk scores in all CHB patients. Different levels of care and different intensities of HCC surveillance should be offered according to the risk profile of patients. Patients at risk of HCC should undergo regular HCC surveillance, even when they are receiving antiviral treatment. Patients who remain at low risk may have less intensive, e.g. 12-monthly or even less frequent, HCC surveillance. Patients who were initially at risk but have their risk down-staged after treatment should continue to be surveyed regularly.
This risk-stratified approach has been the practice in my hospital since 2014 as the resources for USG examination as part of the HCC surveillance program is somewhat limited in this public hospital. Doctors and patients were often surprised when they got an USG appointment scheduled for a few years later, due to limited resources. This is obviously suboptimal if all patients, whether at high risk or low risk of HCC, are to be surveyed every few years. After liaising with our radiologists based on the latest evidence on HCC risk prediction, USG appointments for HCC surveillance are currently scheduled according to their risk scores. Patients of risk scores ranging from 0 to 4 are at low risk of HCC so they would be offered a routine appointment in a few years; those of scores 5 or above are at high risk of HCC and they would be offered an early appointment in 6 to 12 months.
We have to bear in mind that HCC risk score is dynamic; it may decrease after antiviral treatment, it may also increase with increasing age and natural progression of disease in untreated patients. Therefore, the scores should be regularly reviewed and the frequency of surveillance adjusted accordingly. Patients at persistently low risk even under the age of 50 may be also taken care of by family physicians instead of hepatologists. All these measures would result in more efficient employment of the limited health care resources.
Dr. Grace Lai-Hung Wong MBChB(Hons), MD, MRCP, FHKCP, FHKAM(Medicine)
Associate Professor, Divison of Gastroenterology and Hepatology
Department of Medicine and Therapeutics
The Chinese University of Hong Kong
9/F Prince of Wales Hospital
Shatin, Hong KongEmail: [email protected]
Interview with Prof. George Papatheodoridis MD, PhD
Professor in Medicine and Gastroenterology
Director of Academic Department of Gastroenterology, Athens University Medical School, Laiko General Hospital, Athens, Greece

Q: Does hepatocellular carcinoma (HCC) represent a major problem in European chronic hepatitis B (CHB) patients?
Response
“Unfortunately, HCC remains a major problem in European and in Caucasian patients with CHB in general. Although HCC risk may be lower in Caucasian than Asian CHB patients, HCC can develop in all CHB patients regardless of origin. The risk of HCC is particularly high in patients with cirrhosis but HCC may also develop in non-cirrhotic CHB patients. Even patients who have been treated effectively may develop HCC and therefore should be carefully monitored.”
Q: Is there any positive impact on the HCC risk in CHB patients from the available treatment options?
Response
“The data in European and Caucasian patients have not given conclusive results. Regarding interferon-alfa therapy, the existing data come from non-randomised cohort studies using questionably matched historical controls and show that there is some trend for reduction of HCC incidence in interferon-treated patients, but statistical significance cannot be reached. Regarding the oral antivirals that are currently used by the majority of CHB patients, a controlled study in European or Caucasian patients has not been done with the aim to assess the possible impact of therapy on HCC risk. In cohort studies including only Caucasian patients who were treated with the current first-line oral antivirals, entecavir or tenofovir, the mean annual HCC rates have been reported to range widely from 0.1% to 1% in non-cirrhotic and from 1.5% to 5.4% in cirrhotic patients. Given the significant heterogeneity of the studies and the great differences in the patient characteristics between previously reported cohorts of untreated patients and the currently reported cohorts of treated patients with CHB, it is rather difficult to draw definite conclusions. In particular, a major difference between the current and previous cohorts of CHB patients is that current patients are much older (approximately 10-15 years) which puts them at a higher HCC risk.”
Q: Since the HCC risk remains even in treated European CHB patients, do we need to have all these patients under HCC surveillance or may we stratify them according to their HCC risk?
Response
“HCC surveillance is of great importance for European CHB patients, but there are certainly subgroups with no or negligible HCC risk who do not need surveillance for HCC. Recently, several HCC risk scores (CU-HCC, GAG-HCC and REACH-B) have been developed and validated in untreated and treated patients from Asia which were designed to identify CHB patients who need HCC surveillance. Unfortunately, these scores were found to offer poor to moderate predictability for HCC development in Caucasian CHB patients under the current treatment options. Thus, it was clear that a different score was required for European patients. In 2014, in collaboration with several centers in Europe, we developed and validated a simple and reliable HCC risk score for Caucasian CHB patients treated with entecavir or tenofovir. This new score called PAGE-B is based on three variables- age, gender and platelets. Using and scoring these 3 common variables, we can identify a subgroup of CHB patients without any HCC risk, at least within the first 5 years of entecavir or tenofovir therapy. The patients’ follow-up will continue beyond 5 years, when the overall HCC incidence seems to decrease. Thus, a risk-stratified approach for HCC surveillance can be used in European patients with CHB. Such an approach will not only be much more cost-effective for health systems but will decrease the unnecessary procedures for some patients, minimizing their anxiety for potential complications from these. In clinical practice, many hepatologists might have been following a risk-stratified approach for HCC surveillance for years using reasonable clinical judgment based on well-known HCC risk factors such as age and gender. In fact, PAGE-B, the HCC risk score we developed through advanced statistical methodology, combines and provides numerical and objective weight to well known HCC risk factors (age and gender) and is an objective marker of liver disease severity (platelets) without requiring the diagnosis of cirrhosis that may not always be straightforward, especially if liver biopsy is not performed.”
Prof. George Papatheodoridis, MD, PhD
Professor in Medicine and Gastroenterology
Director of Academic Department of Gastroenterology, University Medical School, Laiko General Hospital,
17 Agiou Thoma str.
115 27 Athens, GreeceE-mail: [email protected]
1. BACKGROUND: NECESSITY AND CAUSE
Defined as the MISSION 2020, the main objectives of the Hepatitis Prevention, Control, and Elimination (HPCE) Program in Mongolia are straightforward yet very ambitious:
- To eliminate cancer-causing hepatitis C virus in Mongolia by 2020
- To reduce mortalities related to liver cirrhosis and liver cancer by 50% in Mongolia by 2020
Prevention, early diagnosis and treatment of infected patients are three pillars of infectious disease control. Viral hepatitis is not only an infectious disease, but also it is the main cause of deadly liver cirrhosis and liver cancer. In 2010, liver cancer was the third leading cause of cancer mortalities globally. It was also reported that hepatitis B (HBV) and C (HCV) together killed 1.285 million people in 2010, which is more than number of mortalities due to malaria or tuberculosis (1).
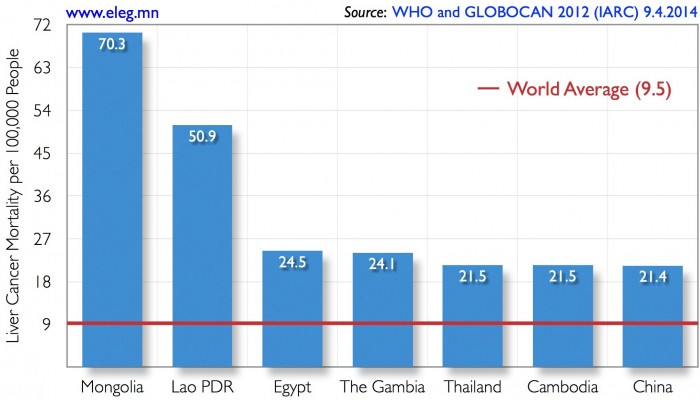
Mongolia has the world’s highest rate of liver cancer mortality — nearly eight times the global average (Fig. 1). Prevalence of chronic viral hepatitis B, C, and D (HDV) in Mongolia is at an endemic level and constitutes the main cause for Mongolia’s world-leading liver cancer mortality rate, which has been steadily increasing over the last decade.
At the moment, liver cirrhosis and liver cancer mortalities account for 15% of all annual mortalities in Mongolia, and it is projected to increase in the future. In short, the viral hepatitis endemic is wreaking havoc in Mongolian society, and it hits very close to home. Our uncles died of liver cancer at young ages of 51 and 57 and our parents have chronic viral hepatitis infections. One of our best friends, a college classmate has HBV and HDV superinfection, leading to an accelerated liver damage that will probably prompt him to get liver transplantation soon. Such heartbreaking tragedies in our personal lives and in Mongolian society compel us to do whatever we can to help to mitigate the endemic of viral hepatitis. Deficiencies in the Mongolian health care system for prevention, early diagnosis and control of chronic conditions compound the endemic. If we can help to prevent even one person from having a liver cancer via improved prevention, early diagnosis, and treatment for viral hepatitis, it means the world to us. It is not only meaningful to us, it’s personal. With this determination, we are implementing the HPCE Program by bringing breakthrough innovations and technologies, industry cooperations, and novel implementation approaches together to achieve the MISSION 2020 of eliminating a major cancer- causing infectious disease in a country for the first time in world history and reducing disproportionate, sustained burden of liver cirrhosis and liver cancer mortalities significantly in a country with the highest rate of liver cancer mortality in the world.
2. MISSION AND EXPECTED RESULTS
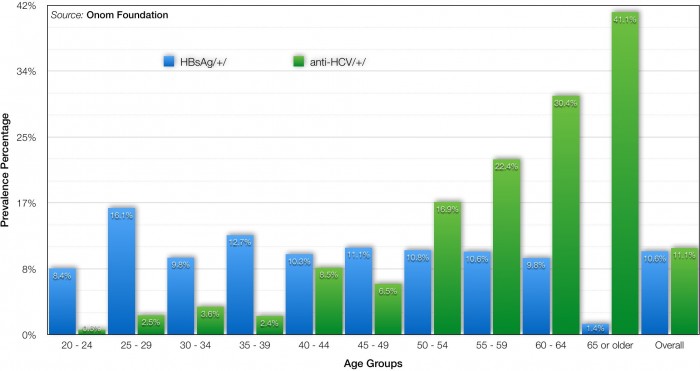
Is the MISSION 2020 attainable? Based on solid scientific findings, facts, and evidences, we confidently infer that the MISSION 2020 is indeed attainable. In our epidemiological study that determined the prevalence of viral hepatitis in Mongolia in 2013, we discovered that there is a cohort effect for chronic HCV wherein some high risk factor in the past is no longer occurring, which in turn means that a probability for re-infection of HCV after getting treatment is less pronounced (Fig. 2). Based on this scientific finding, we conclude that it is practically feasible to eliminate HCV in Mongolia by utilizing revolutionary new HCV treatments.
In addition, using the age distribution of the viral hepatitis prevalence and population statistics, we estimate that roughly 115K people have chronic HCV infection, while roughly 200K people have chronic HBV infection in Mongolia. These population sizes are manageable. In other words, with relatively small viral hepatitis prevalent population sizes combined with the cohort effect for HCV infection, Mongolia might be the only country where viral hepatitis is highly prevalent and causes devastating effects on its society, yet it is possible to eliminate HCV and to control HBV in relatively short amount of time. In fact, according to hepatitis treatment simulation study that we published in January 2015 issue of the Journal of Viral Hepatitis (2, 3, and 4) along with colleagues, elimination of chronic HCV in Mongolia can be achieved by 2020 if roughly 15K patients treated annually starting in 2015.
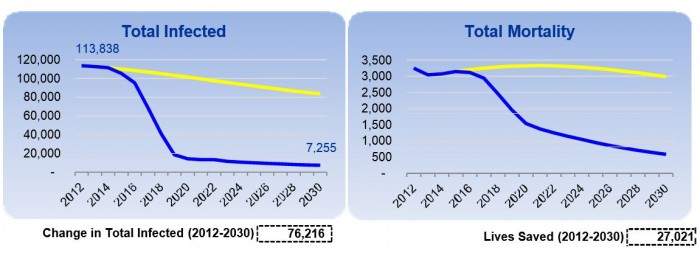
According to this treatment dynamics model, the total number of HCV infected would decline by 91% in 2030 and over 27,000 lives (Fig. 3) will be saved from dying of liver cirrhosis or liver cancer by 2030 in the process of eliminating HCV in Mongolia. These results are truly remarkable and demonstrate the true impact of the HPCE Program in making a difference in lives of ordinary Mongolians. Finally, working with the Onom Foundation and its partners, you can set a shining example in eradicating viral hepatitis for the whole world to follow suit since the elimination of HCV and control of HBV and HDV in Mongolia will serve as a model for other countries in the world with high prevalence and burden of viral hepatitis.
3. IMPLEMENTATION OVERVIEW
The Hepatitis Prevention, Control, and Elimination Program in Mongolia is a comprehensive national program that is divided into three main intrinsically inter-dependent campaigns with specific focuses on prevention, early diagnosis, and treatment:
Prevention Campaign—Nationwide public health campaign will be conducted to increase awareness and knowledge of Mongolian public about viral hepatitis and its deadly consequences and ways of preventing viral hepatitis infection and protecting loved-ones from this life-threatening disease. Analysis of data from public knowledge and awareness assessment surveys will be used to regularly optimize the design and execution of the public health campaign. In addition to the public health campaign, HBV vaccination of high-risk groups such as healthcare workers and emergency responders will be intensified around Mongolia. In fact, the plan is to vaccinate all healthcare workers and other high-risk groups in Mongolia against HBV and install a system in place to continue the vaccination. With these measures, prevalence of HBV and HDV will be controlled and ultimately lowered in Mongolia.
Screening and Early Diagnosis Campaign—Identifying and registering these people with chronic hepatitis infections will be the key to achieving the MISSION 2020. Therefore, we are conducting decentralized nationwide screening for viral hepatitis infection, to identify and register HBV and HCV infection status of everyone in Mongolia using inexpensive, on-site, rapid tests that can be performed at the 567 primary and secondary healthcare facilities throughout Mongolia. Once hepatitis testing is performed, the test results will be sent via SMS message to the Mobile Messaging Platform along with individual’s name and national identification number. To demonstrate its feasibility, we have already piloted this system and have 5,806 valid registrations into the National Viral Hepatitis Database as of January 12, 2015. In other words, the power of crowdsourcing will be used to register every individual tested for viral hepatitis infections into the National Viral Hepatitis Database via the Mobile Messaging Platform. Blood samples of people with viral hepatitis infections will be collected using new class of dry blood transportation system for further quantitative testing at central laboratories.
Treatment Campaign—Thanks to industry cooperation for the HPCE Program, the most effective and breakthrough medicines for chronic viral hepatitis will be introduced in Mongolia with over 98% discount of the world market price. As part of the treatment campaign, over 110,000 people with chronic HCV will be cured by 2020. Over 100,000 people with chronic HBV will be enrolled into antiviral treatment regimen. As a result, mortalities related to liver cirrhosis and liver cancer in Mongolia will be reduced by 50% by 2020 and cancer-causing HCV will be eliminated in Mongolia.
On top of these campaigns, we are also establishing a comprehensive research program to investigate a number of aspects ranging from health economics to clinical trials.
REFERENCES
- Lozano R et al. 2012. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study Lancet 380(9859): 2095-2128.
- Saraswat V et 2015. Historical Epidemiology of Hepatitis C Virus (HCV) in Select Countries – Volume 2. Journal of Viral Hepatitis 22(Suppl. S1): 6-25.
- Hatzakis A et 2015. The Present and Future Disease Burden of Hepatitis C Virus (HCV) Infections with Today’s Treatment Paradigm – Volume 2. Journal of Viral Hepatitis 22(Suppl. S1): 26-45.
- Gane E et 2015. Strategies to Manage Hepatitis C Virus (HCV) Infection Disease Burden – Volume 2. Journal of Viral Hepatitis 22(Suppl. S1): 46-73.
An interview with Ingrid Hickman AdvAPD, PhD, Director of Research, Department of Nutrition and Dietetics, Alexandra Hospital and the University of Queensland, Brisbane, Australia.
Q: Are the causes of NASH known and what is the disease burden?
Response
“ It is still unclear why some people with fatty liver go on to develop the more severe form NASH while others do not, but it is almost certainly multifactorial. The increasing prevalence of obesity and type 2 diabetes is associated with the increasing prevalence of fatty liver and there is a greater risk of NASH if you are obese and have type 2 diabetes. NASH is predicted to become the most common reason for liver transplantation in the future.”
Q: Is there any evidence that onset and prognosis of NASH are related to lifestyle?
Response
“Yes. Patients with NASH are often overweight and lead sedentary lifestyles. We know that if weight loss occurs through reducing calorie intake and also increasing physical activity, that significant improvements in liver tissue can occur which may result in a better prognosis. Sweetened beverages such as soft drinks and fruit juices can increase liver fat remarkably and have been associated with increased fibrosis in adults and increased incidence of fatty liver in obese adolescents.“
Q: Is there any validated effective drug to prevent and effectively treat NASH?
Response
“No. Lifestyle intervention is the primary treatment option for NASH.“
Q: Do diet and exercise favourably impact on NASH?
Response
“Yes. In combination, resulting in weight loss, they have demonstrated to improve insulin resistance and decrease fatty liver and liver injury. But, even in the absence of weight loss, beneficial changes to diet such as embracing the Mediterranean style of eating, can significantly improve liver fat and metabolic factors. Exercising regularly will improve body composition such as reducing visceral fat and increasing muscle mass, and improves fitness and cardiovascular risk factors. Patients with NASH should be encouraged to reduce calories in order to lose around 7% body weight, choose lower carbohydrate and higher monounsaturated fat foods, and exercise daily.”
Ingrid Hickman AdvAPD, PhD
Director of Research
Department of Nutrition and Dietetics
Alexandra Hospital
Honorary research fellow
Mater Research Institute
The University of Queensland
Brisbane
Australia
Email: [email protected]